Production process (the mechanism of photocatalysis)

photosynthesis of plants."
Akira Fujishima
In 1972 the postgraduate student of the University of Tokyo Akira Fujishima and his scientific adviser Professor Kenichi Honda searched for new technologies of hydrogen production. An article in the prestigious journal "Nature" was the result of their work. The process of water decomposition on the surface of titanium dioxide (TiO2) crystals under the impact of sunlight has been described in this article for the first time.
The process was named “photocatalysis”. In 1985, again in Japan, Takehiro Matsunaga just joined a post-graduate course of Tokyo University, reported about bactericidal abilities of TiO2 to the world. Since then hundreds of scientific papers on photocatalysis were written.
So what is photocatalysis? Simply speaking it is the acceleration of a chemical reaction associated with the joint action of the catalyst and light.
On this video you can see the phenomenon of photocatalysis in practice.
During 6 hours the tobacco tar applied to the photocatalytic element is completely decomposed into water and carbon dioxide under the UV-radiation.
UV-radiation
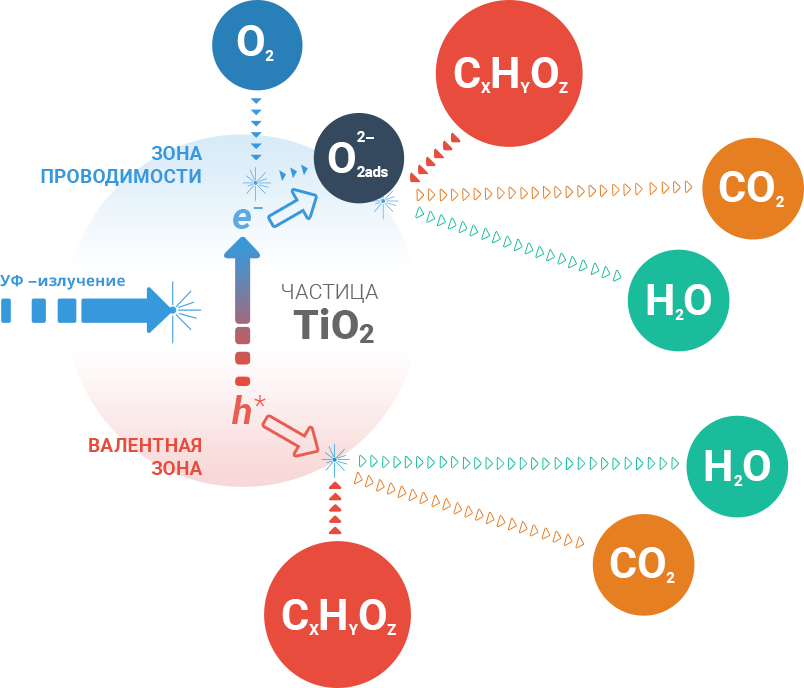
The reaction of photocatalysis in detail
TiO2 is a semiconductor. In such compounds the electrons can be in two states: free and bound. Normality of the electron is bound, i.e. it is associated with the ion of crystal lattice substance forming a strong chemical bond.
It is necessary to apply more than 3.2 electron volts (eV) energy to "pull out" the electron from the lattice (compare: the kinetic energy of a flying mosquito is about a trillion eV). To our joy, that is the amount of energy a light quantum carries with a wavelength less than 390 nM. So, the quantum of light "knocks" an electron from the lattice forming an electronic vacancy or simply "the hole."
The electron and the hole are moving actively inside the TiO2 particles. As a result of the movement they are either recombined (meet each other, "marry each other" and returned in a bound state) or erupt on the surface and immediately are captured by it.
Both the hole and the electron are incredibly reactive. All-over surface catalyst is a powerful field of oxidation. The oxygen contacting the catalyst surface receiving a free electron as a gift, gives rise to oxidative radical O-, which is able to destroy (oxidize) any organic compound. Hole in turn reacts with the first organic compound encountered on the surface. Hole pulls out from the connection structure missing her electron, thereby resolving the compound into water and carbon dioxide.
Every time replaced “used-up” pairs electoron-"hole" rises on the catalyst surface like bubbles in a champagne glass, new pair free. The oxidation process will go on until the light is incident on the catalyst.
mineralises molecular organic impurities
primarily to carbon dioxide
and water
Our technological innovations:
Catalyst. TiO2 with crystalline modification of anatase and containing minimum impurities has the highest photocatalytic activity. In our devices we use such catalyst in the form of ultrafine powder of own production. A particle size is about 40 nm. Just in this state the catalyst is highly active and has a maximum surface for the reaction.
The carrier (the structural component which surface is covered withTiO2). The carrier material requirements are quite rigorous: it can't be made of organic materials as any organic matter decomposes under the ultraviolet radiation, it needs to transmit ultraviolet which means that should be transparent and lastly it must be small size but has a huge surface to contact the catalyst and air. We know how to make such carriers obtaining plates or tubes from sintered quartz beads 1 mm in diameter by own patented technology.
1 mm. diameter
TIOKRAFT schematic diagram
-
1
Pre-filter removes coarse-dispersed dust from the cleaned air.
-
2
Fan provides the flow of cleaned air through the device.
-
3-4
Electrostatic filter retains the smallest aerosol particles, bacteria, viruses and mold spores.
-
5-6
Photocatalytic filter mineralizes organic molecular impurities to carbon dioxide and water. It consists of UV-lamps operating in the "soft" range radiation (320-405 nm) and the photocatalytic element on inorganic basis.
-
7
Coal-catalytic filter prevents the overshoot of hazardous substance due to their adsorption on the surface of the charcoal adsorbent. The collaboration technology of adsorption and photocatalytic filters allows us to make an adsorbent regenerable with the increase of the service life up to 10 times.
-
8
Cleaning unit from carbon monoxide contains a Pt/Pd catalyst on nano-crystalline carrier. In addition to carbon monoxide it removes from the air low-molecular organic compounds (Option).
-
9
Power pack and alarm system include a sensor system of automatic control (VR400A only) that set the operation mode of the air cleaner depending on molecular concentration of organic pollutants level in the clean air.